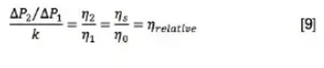Conventional Rheology
|
The conventional definition of viscosity is a ratio of the pressure exerted on a material divided by the rate of shear on the material. This is the fundamental principle upon which the science of rheology is built. While shear effects, temperature effects, and elastic effects of the polymer are important considerations which measuring solution viscosity, they are variables to be controlled, rather than the basis of the science of dilute solution viscosity.
|
Dilute Solution Viscosity
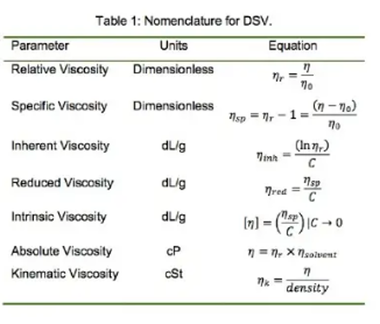
Dilute Solution Viscosity (DSV) is the viscosity measurement of dilute solutions of polymers. Typically, a sample is dissolved in a solvent at a specified concentration in the range of 0.2 to 1.0 g/dL. Polymer solution viscosity is measured relative to the viscosity of the pure solvent. The relative viscosity (ηrelative) is simply the ratio of the two measurements: Where η is the polymer solution viscosity and η0 is the viscosity of the pure solvent.
Traditional DSV Measurement
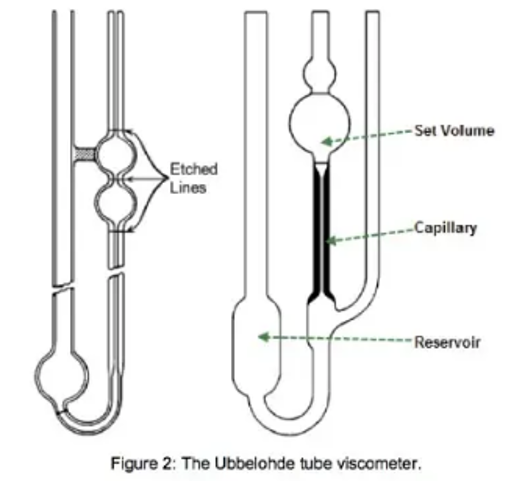
With a traditional glass capillary tube or Ubbelohde tube (Figure 2) viscometer, we measure the time it takes for the test liquid to flow through a capillary of a known diameter between 2 marked points.
Solvent is placed into the tube with enough volume to fill the reservoir. The user then draws the solvent into the set volume compartment, and marks the time taken for the solvent to drop from one set point to the next. The experiment is then repeated with the polymer solution.
Solvent is placed into the tube with enough volume to fill the reservoir. The user then draws the solvent into the set volume compartment, and marks the time taken for the solvent to drop from one set point to the next. The experiment is then repeated with the polymer solution.
If t = polymer drop time, and t0 = solvent drop time:
|
Determining viscosity in this manner is both time-consuming and error-prone. The glass capillary tube returns a kinematic viscosity, which is viscosity divided by density. In situations where density is a variable, this then requires an extra measurement of density to calculate a true relative viscosity. Temperature control and cleanliness are key to a precise result.
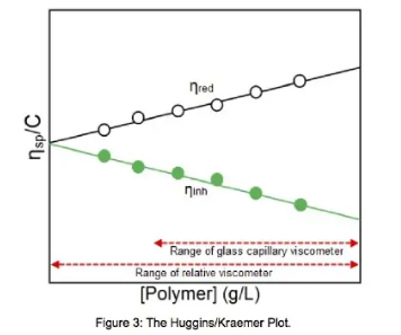
In addition, glass capillary viscometers cannot operate at very low concentrations and must therefore use several concentrations for a Huggins plot (see Figure 3) to derive intrinsic viscosity by extrapolation to zero concentration. The HMJ Relative Viscometer is able to operate at low concentrations and will give intrinsic viscosity from one sample run by use of the Solomon-Gatesman equation. |
Intrinsic Viscosity
Intrinsic viscosity is the volume per unit mass that the polymer occupies in a solution, and so it is the inverse of molecular density. Intrinsic viscosity is not viscosity; the units are in dL/g, and viscosity is measured in Pa*s. It is directly related to molecular weight by the Mark-Houwink equation:
where [η] = Intrinsic Viscosity, k = Mark-Houwink constant, a = Mark-Houwink constant relating to structure (0 to 0.1 – sphere, 0.35 to 0.80 – random coil, 1.5 to 2 – rigid rod), and Mv = viscosity average molecular weight.
HMJ Relative Viscometer Principle
The proper tool to measure relative viscosity and all of the solution viscosity values calculated from the relative viscosity is naturally a relative viscometer. The solution relative viscosity is measured directly by analyzing both solvent and sample viscosity simultaneously, avoiding errors due to temperature fluctuations and solvent variations.
The dual capillary viscometer works on the basic principle of Poiseulle’s Law [6], which states that the pressure drop across a capillary is equal to the viscosity of the material multiplied by the flow rate multiplied by the resistance of the capillary (which is defined by the length and diameter of the capillary).
The dual capillary viscometer works on the basic principle of Poiseulle’s Law [6], which states that the pressure drop across a capillary is equal to the viscosity of the material multiplied by the flow rate multiplied by the resistance of the capillary (which is defined by the length and diameter of the capillary).
where ΔP = pressure drop, η = viscosity, Q = flowrate, and R = resistance.
The two capillaries are in series so that a ratio measurement can be made of the solution and the sample simultaneously. The pressure ratio is therefore:
The two capillaries are in series so that a ratio measurement can be made of the solution and the sample simultaneously. The pressure ratio is therefore:
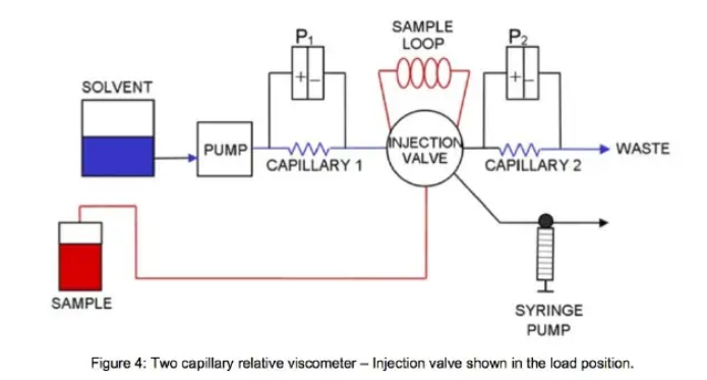
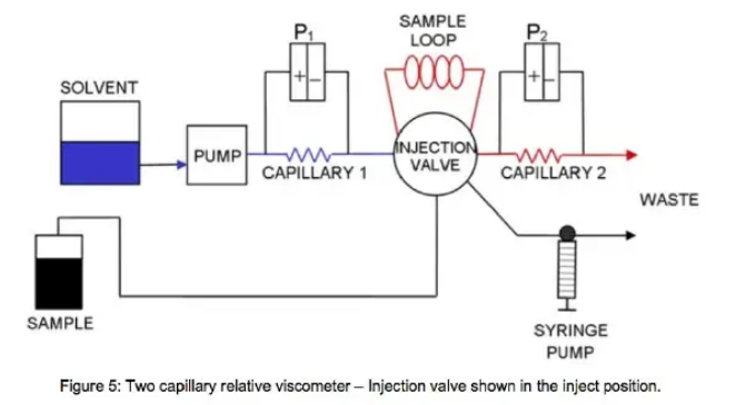
The relative viscometer is shown in both the load and bypass position in Figure 4 and 5 respectively.
Under baseline conditions, pure solvent flows through both R1 and R2. Therefore, viscosities in both capillaries are equal and cancel out. Since the capillaries are in series and the liquid is non-compressible, the flow rate Q1 and Q2 are also equal and cancel. This leaves:
where k is the instrument constant.
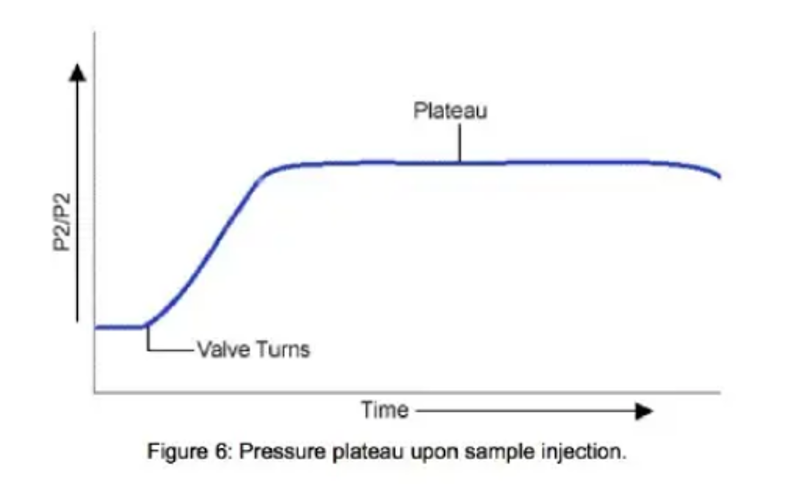
When the sample is injected into the sample loop (Figure 5), the solvent is diverted from the sample loop bypass route and enters the sample loop to push sample across capillary 2, at which time the pressure on the transducer will reach a maximum or plateau (Figure 6)
When the sample is injected into the sample loop (Figure 5), the solvent is diverted from the sample loop bypass route and enters the sample loop to push sample across capillary 2, at which time the pressure on the transducer will reach a maximum or plateau (Figure 6)
When the sample is injected, we know that the pressure ratio is still governed by [7]. The ratio of R2 to R1 was previously measured as an instrument constant. Although the flow path now includes the sample loop, the capillaries are still in series, and so Q1 and Q2 are equal and cancel. The equation for relative viscosity reduces to:
Summary
The relative viscometer:
- Measures the relative viscosity directly in experiment.
- Matches the temperatures of the sample and solvent because the measurements are done in the same enclosure
- Automatically cleans the sample capillary with a plug flow of solvent when the valve is turned back to the load position
- Calibrates the capillaries between each injection by measuring g the baseline ratio. Any partial plugging is measured and compensated for.
- Can control the shear rate on the solution for polymers that are shear sensitive.